The Ultimate Guide to Hyaluronidase Brands: A Professional Comparison
In aesthetic medicine, **hyaluronidase** is the essential reversal agent for hyaluronic acid (HA) dermal fillers. This enzyme, critical for managing complications like vascular occlusion or overcorrection, demands a deep understanding of the available brands. This comprehensive guide breaks down the market’s leading hyaluronidase products, comparing their technical specifications, origins, and clinical profiles to help practitioners make informed choices. We focus on the crucial distinctions between budget-friendly and premium options, specifically looking at source material, purity, and the critical factor of immunogenicity (allergic reaction risk).
🔬 Understanding Professional Hyaluronidase: Quality and Key Features
The quality of a professional-grade hyaluronidase product is determined by several core characteristics that directly impact its safety and efficacy in a clinical setting. Aesthetic practitioners must prioritize these features over simple cost.
1. Source and Purity: Recombinant Human vs. Animal-Derived
- **Recombinant Human Hyaluronidase (rHuPH20):** This is synthesized using recombinant DNA technology, resulting in an enzyme structure identical to the human body’s naturally occurring hyaluronidase. **Key Advantage:** It boasts the highest purity and significantly lower risk of allergic reactions (immunogenicity) compared to animal-derived products.
- **Animal-Derived Hyaluronidase (Bovine or Ovine):** Sourced from the testes of cows (bovine) or sheep (ovine). **Key Disadvantage:** These preparations are less pure and contain non-human proteins, which increases the likelihood of immediate or delayed hypersensitivity reactions.
2. Concentration and Activity (IU/mL)
Hyaluronidase is measured in International Units (IU), which quantify the enzyme’s capacity to degrade hyaluronic acid. Professional products typically range from 150 IU/mL to 1,500 IU per vial. The concentration required depends heavily on the clinical indication, such as treating a vascular occlusion (requiring a high, immediate dose) versus correcting a simple overfill.
3. Stability and Storage
Many hyaluronidase preparations, especially those in liquid form, require strict cold-chain storage (refrigeration) to maintain potency. Lyophilized (freeze-dried) powder formulations offer greater shelf stability at room temperature but require precise reconstitution, adding a procedural step.
💰 Budget-Friendly Hyaluronidase: High-Value Options
The “budget-friendly” segment often comprises brands that, while effective, are typically derived from animal sources or are internationally compounded products, offering a cost advantage. They are often chosen for non-emergency corrections where a skin patch test may be feasible.
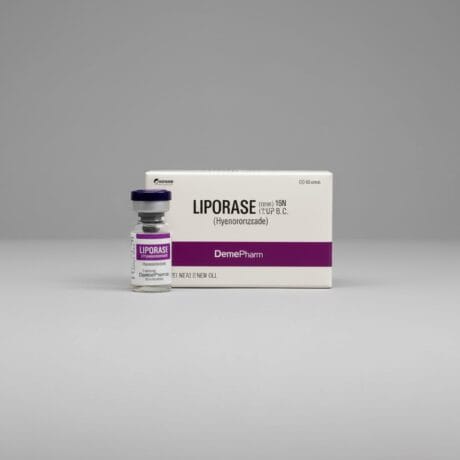
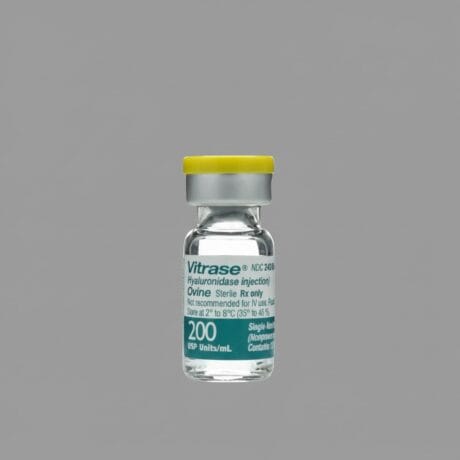
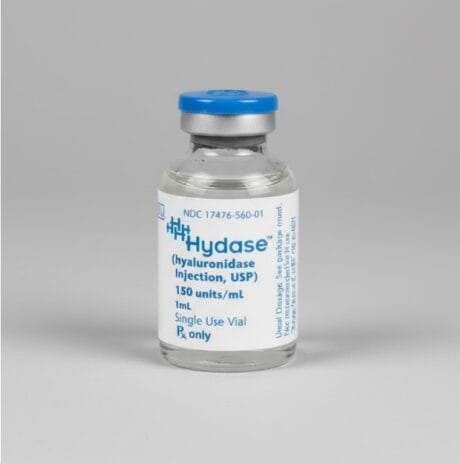
| Product 1: **Liporase** (Compounded/Korean) | Product 2: **Vitrase** (Ovine Source) | Product 3: **Hydase** (Bovine Source) |
|---|---|---|
 |  |  |
| Source/Type | Source/Type | Source/Type |
| Animal-Derived (Often Ovine/Compounded) | Ovine (Sheep) Hyaluronidase | Bovine (Cow) Hyaluronidase |
| Standard Concentration | Standard Concentration | Standard Concentration |
| 1,500 IU per vial (Lyophilized Powder) | 200 IU/mL | 150 IU/mL |
| Allergic Risk Profile | Allergic Risk Profile | Allergic Risk Profile |
| Higher (Animal Source/Purity Varies) | Moderate to High (Animal Protein) | Moderate to High (Animal Protein) |
| Professional Use Note | Professional Use Note | Professional Use Note |
| Popular for large-volume dissolution; always require sterile reconstitution. | Effective, but necessitates pre-treatment screening for sensitivity. | A traditional option, often used where rHuPH20 is not financially viable. |
| Buy Now | Buy Now | Buy Now |
Final VERDICT (Budget Comparison)
For practitioners balancing efficacy with cost, **Liporase** is widely utilized globally due to its high concentration (1,500 IU) per vial, making it cost-effective for larger corrections. However, its high variability in sourcing and purity, and the need for reconstitution, make **Vitrase** and **Hydase** slightly more controlled options in regulated markets, despite the persistent (though low) risk of animal-protein-related allergic reactions.
💎 Premium Hyaluronidase: Safety and Purity
The premium category is defined by the use of recombinant human hyaluronidase (rHuPH20), which offers the highest level of safety and purity. These products are the industry standard for time-sensitive, high-risk procedures like vascular occlusion, where minimizing the risk of an adverse allergic event is paramount.
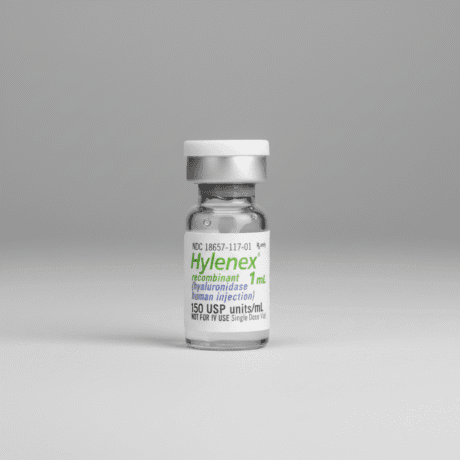
| Product 1: **Hylenex** (Recombinant Human) | Product 2: **Amphadase** (Bovine Source – High Purity) | Product 3: **Hyalase Desau** (Bovine/Compounded – EU) |
|---|---|---|
 |  |  |
| Source/Type | Source/Type | Source/Type |
| **Recombinant Human** (rHuPH20) | Bovine (Cow) Hyaluronidase | Bovine (Cow) Hyaluronidase |
| Standard Concentration | Standard Concentration | Standard Concentration |
| 150 IU/mL (Liquid) | 150 IU/mL (Liquid) | 300/1,500 IU (Lyophilized Powder) |
| Allergic Risk Profile | Allergic Risk Profile | Allergic Risk Profile |
| **Lowest Risk (Human Recombinant)** | Low to Moderate (Regulated Purity) | Moderate (Requires Reconstitution) |
| Professional Use Note | Professional Use Note | Professional Use Note |
| The gold standard for safety, essential for emergency vascular occlusion protocols. | FDA-approved animal product, offering a highly regulated quality level. | EU-popular brand, known for its high-dose powder formulation flexibility. |
| Buy Now | Buy Now | Buy Now |
Verdetto Finale (Premium Comparison)
The clear leader in the premium category is **Hylenex** due to its recombinant human source. The significantly reduced risk of immunogenicity makes it the definitive choice for critical and emergency procedures, where a delay due to an allergic reaction is unacceptable. While Amphadase is highly regulated, Hylenex offers superior biological compatibility, justifying its position as the top-tier choice for safety-conscious practices.
Detailed Professional Product Profiles
✨ Hylenex (Recombinant Human Hyaluronidase)
Hylenex, or Hyaluronidase Human Injection, represents the zenith of hyaluronidase technology. Developed using recombinant DNA methods, its enzyme structure is identical to that naturally found in human tissue (rHuPH20). This crucial biological feature confers the highest possible safety profile, making it the **undisputed gold standard for emergency vascular occlusion management.** Unlike its animal-derived counterparts, Hylenex carries the lowest risk of immediate or delayed allergic reactions, eliminating the need for routine skin patch testing and allowing for faster, safer intervention in critical situations. The product is typically presented in a stable liquid form with a concentration of 150 IU/mL, though it can be diluted or used in higher concentrations as needed. Its premium positioning reflects the superior manufacturing purity and the associated decrease in patient risk. Practitioners rely on Hylenex for its predictable performance and immediate availability, particularly in protocols addressing acute complications. Its primary drawback remains the higher cost per vial compared to bovine or ovine products, a factor often considered negligible when weighed against the patient safety benefits and regulatory compliance it provides. The stability, consistency, and unparalleled safety record solidify Hylenex’s status as the definitive choice for top-tier aesthetic clinics globally, where patient well-being is the ultimate priority. The use of a human-identical enzyme ensures optimal diffusion and breakdown of HA fillers, leading to rapid and reliable reversal of adverse events.
💸 Liporase (Compounded/International Hyaluronidase)
Liporase has gained significant traction in the aesthetic field, particularly in international markets, largely due to its high unit concentration and corresponding cost-efficiency. Typically sold as a lyophilized (freeze-dried) powder, it offers a substantial 1,500 IU per vial, making it exceptionally economical for practitioners dealing with large volumes of filler to dissolve, or for non-emergency corrections. The active enzyme is generally a bovine or ovine-derived hyaluronidase. However, the product is often marketed without the stringent quality controls and regulatory oversight of FDA or EMA-approved brands, which is the primary reason for its lower price point. This raises a crucial professional consideration: the purity and consistency of the enzyme can vary. Being an animal-derived powder, it requires proper sterile reconstitution by the clinician, which adds a crucial step to the procedure that must be performed flawlessly to maintain product integrity and safety. While highly effective in dissolving HA, the animal origin means it has an inherently higher risk profile for allergic reactions compared to recombinant human brands. Due to this potential for immunogenicity, many practices utilizing Liporase perform a mandatory skin test before administering the full dose, although this practice is debated. Its efficacy, high potency, and low cost make it a high-value tool for practices looking to optimize supply expenses for routine overcorrection, provided they are willing to accept the marginally higher procedural risk and complexity associated with its source and preparation.
🐑 Vitrase (Ovine-Derived Hyaluronidase)
Vitrase is a long-standing, regulated brand of hyaluronidase sourced from ovine (sheep) testicular tissue. As one of the original regulated products on the market, it has a documented history of use, not only in aesthetics but also for increasing the absorption of other injected drugs. It is typically supplied in a liquid formulation, simplifying the administration process compared to lyophilized powders. The standard concentration of 200 IU/mL provides potent activity suitable for most corrective procedures. Since it is an animal-derived enzyme, Vitrase contains foreign proteins, which necessitates a careful patient history assessment for potential allergies, particularly to bee stings, as there is a documented cross-reactivity risk with some animal-derived hyaluronidases. While not possessing the zero-risk profile of the recombinant human types, Vitrase offers a highly regulated, consistent quality that appeals to clinics operating under strict oversight. Its manufacturing consistency is generally superior to that of unregulated compounded products. For clinicians who prefer a liquid, ready-to-use formulation but find recombinant human products cost-prohibitive for high-volume, routine corrections, Vitrase often serves as a reliable middle-ground. It remains a fixture in many professional clinics, prized for its controlled efficacy and established safety record within the context of non-human source material.
🐄 Amphadase (Bovine-Derived Hyaluronidase)
Amphadase is a prominent, regulated brand of hyaluronidase derived from bovine (cow) testicular tissue. Similar to Vitrase, it is a traditional enzyme preparation known for its robust and reliable activity, typically supplied as a liquid at a concentration of 150 IU/mL. Its key advantage over unregulated budget options lies in its rigorous manufacturing process and regulatory approval, which ensures a consistent and high level of purity for an animal-sourced product. This consistency is paramount in clinical practice, allowing practitioners to rely on predictable outcomes. Amphadase is frequently used in general medical applications, such as hypodermoclysis, cementing its professional utility beyond just aesthetics. In the aesthetic context, it is a powerful tool for dissolving HA fillers, suitable for a wide range of corrections, from localized lumps to larger volumes of misplaced filler. As with all non-recombinant products, the presence of bovine proteins means that the risk of an allergic reaction, though low, is present, mandating careful patient screening and preparedness for anaphylaxis. The product’s primary competitive disadvantage is the presence of recombinant human options like Hylenex, which offer a demonstrably lower immunogenic risk. Nevertheless, Amphadase remains a preferred choice for many established clinics due to its long market history, efficacy, and the confidence that comes from a domestically regulated supply chain. It is a benchmark for quality among animal-derived hyaluronidases.
💧 Hydase (Bovine-Derived Hyaluronidase)
Hydase is another significant, regulated hyaluronidase brand, also utilizing a bovine-derived source. It is functionally very similar to Amphadase and is typically supplied in a liquid form at 150 IU/mL. The distinctions between these regulated bovine products often come down to supplier pricing, regional availability, and specific preservative formulations, rather than core enzymatic differences. Hydase is valued in clinical practice for its ease of use; being pre-mixed, it avoids the contamination risks and reconstitution time associated with powder vials, which is a key factor in time-critical aesthetic corrections. Its bovine source dictates the same cautionary approach as other animal-derived products, requiring an awareness of the slightly elevated risk of hypersensitivity compared to the human recombinant option. However, for clinics where the budget does not permit exclusive use of Hylenex, but where the quality must be higher than unregulated foreign compounds, Hydase provides a safe, FDA-approved, and effective alternative. It offers a balance of reliable efficacy and cost-effectiveness, securing its place in the middle-to-lower end of the professional budget scale. Its established presence in regulated medical settings gives practitioners confidence in its quality control and predictable performance, making it a reliable workhorse for routine filler dissolution procedures.
🇪🇺 Hyalase Desau (High-Dose EU Hyaluronidase)
Hyalase Desau is a well-known hyaluronidase brand in European markets, offering a versatile range of concentrations, often in lyophilized powder form (e.g., 300 IU or 1,500 IU). This powder format, while requiring sterile reconstitution, allows for high-dose flexibility, enabling practitioners to create precisely the concentration needed for specific treatment protocols—a significant advantage in tailored aesthetic care. The enzyme is typically bovine-derived, placing it in the same risk category as other animal-sourced products regarding immunogenicity. Its high-dose variants (1,500 IU) make it particularly popular for addressing large, complicated areas or for body contouring applications where substantial amounts of filler need to be reversed. The reputation of Hyalase Desau is built on its widespread adoption and proven clinical efficacy throughout Europe. However, practitioners outside of its primary market must navigate importation rules and be absolutely meticulous in the reconstitution process to ensure sterility and accuracy of the final injectable concentration. The need for precise preparation contrasts with the ready-to-use nature of liquid products like Hylenex or Amphadase, but its flexibility and concentration options maintain its status as a top-tier choice for many European experts who value tailored dosing capabilities for advanced treatments.
✅ Pros and Cons of Each Brand Type
Choosing between hyaluronidase brands is a trade-off between immunogenic safety, procedural speed, and cost. Here is a simplified Pro/Con table based on the source material.
| Recombinant Human (e.g., Hylenex) | Animal-Derived (e.g., Liporase, Vitrase, Hydase, Amphadase) |
|---|---|
PROS (The Green Light)
| PROS (The Green Light)
|
CONS (The Red Flag)
| CONS (The Red Flag)
|
Conclusion: The Safety-First Approach
For any aesthetic practitioner, stocking hyaluronidase is a mandate of safety, not merely an optional treatment. The choice of brand should ultimately be dictated by the principle of minimizing patient risk. The premium brands utilizing **Recombinant Human Hyaluronidase (Hylenex)** are the optimal choice for this reason. Their superior purity and near-zero immunogenic risk make them indispensable for managing vascular occlusions, where every second counts and a complication must not be compounded by an allergic emergency.
While the budget-friendly, animal-derived options like **Liporase, Vitrase, and Hydase** offer substantial cost savings, practitioners must be acutely aware of the slightly increased risk of hypersensitivity reactions. These can be reserved for less urgent, non-vascular corrections, provided a thorough patient history is taken and emergency protocols are readily available.
Ultimately, a successful aesthetic practice maintains both the **highest quality product (Hylenex)** for emergencies and a detailed understanding of the efficacy and limitations of all available brands to ensure safe and effective filler dissolution.
**Disclaimer:** Hyaluronidase is a prescription medication and should only be handled and administered by licensed and trained medical professionals.